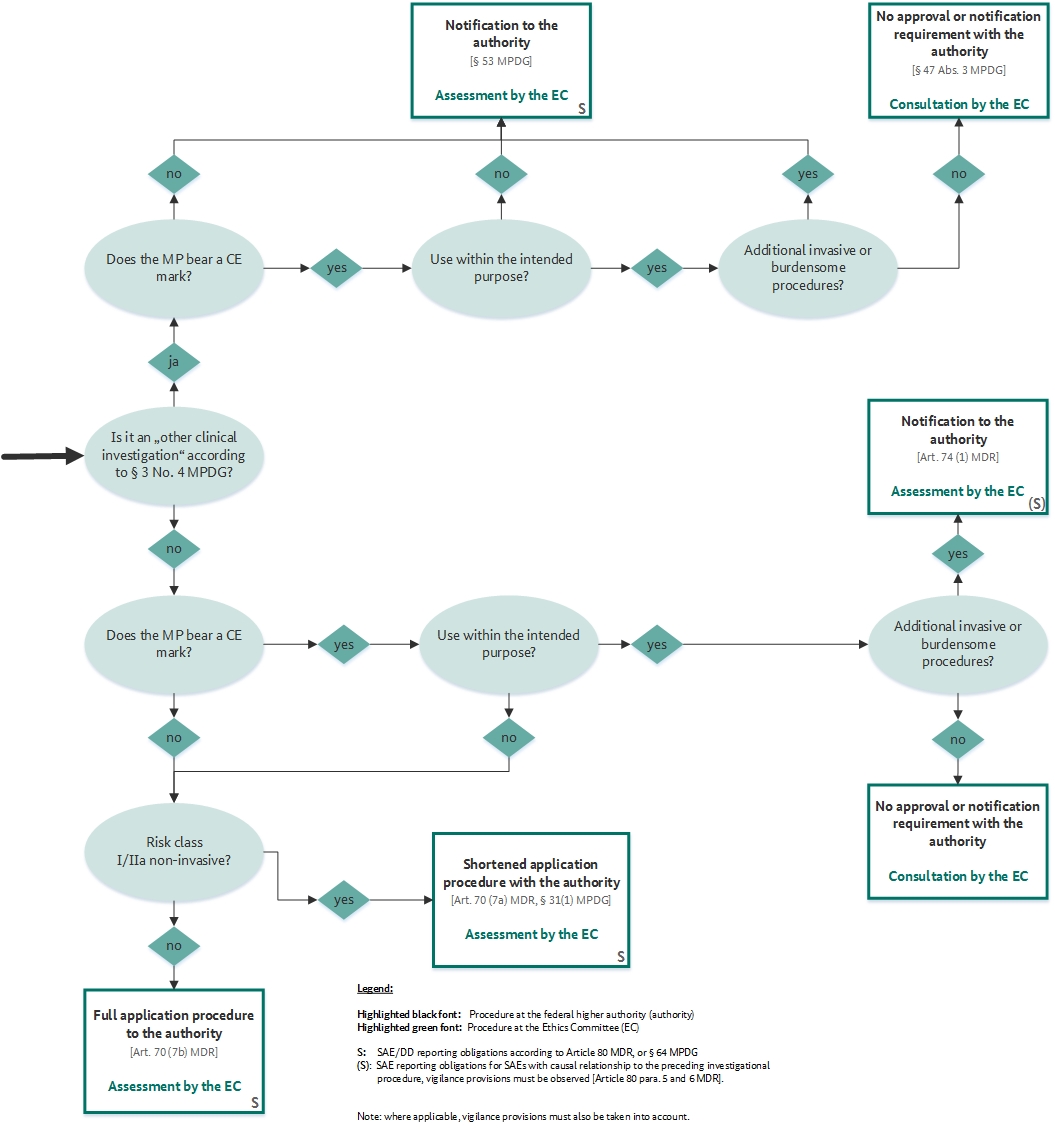
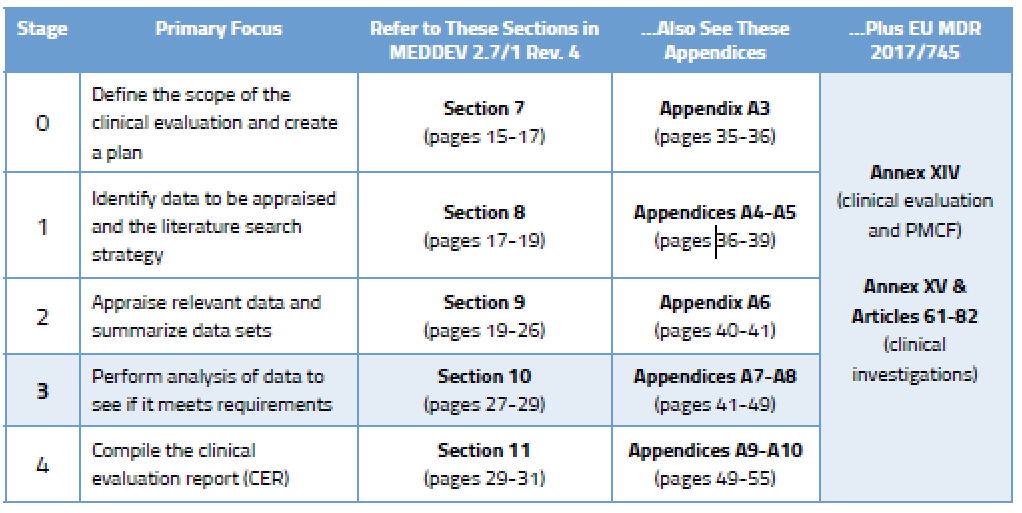
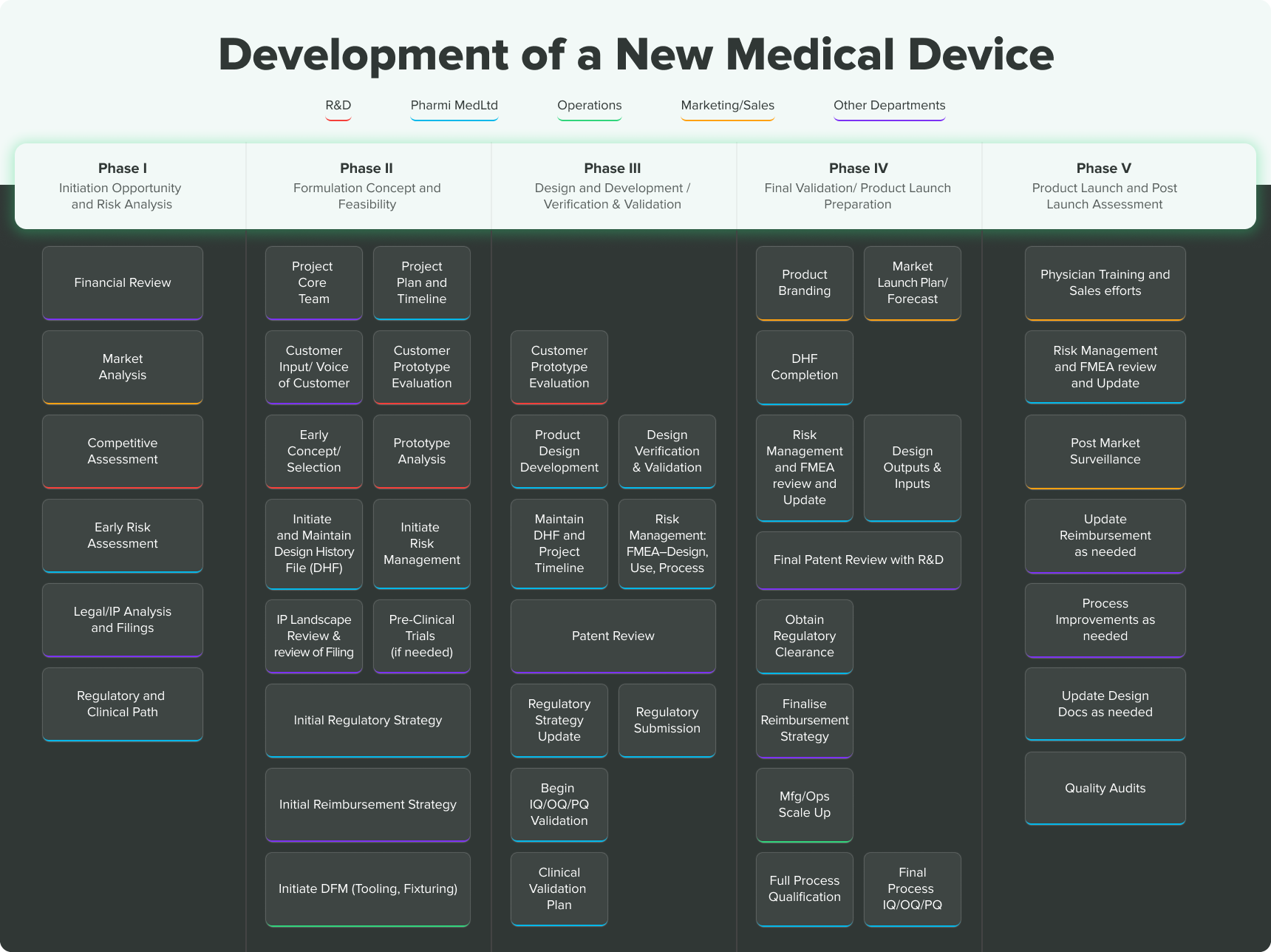
ONORM EN ISO 14155-2:2009 - Clinical investigation of medical devices for human subjects - Part 2: Clinical investigation plans (ISO 14155-2:2003)
Software as a Medical Device (SAMD): Clinical Evaluation - Guidance for Industry and Food and Drug Administration Staff

ONORM EN ISO 14155-2:2003 - Clinical investigation of medical devices for human subjects - Part 2: Clinical investigation plans (ISO 14155-2:2003) (FOREIGN STANDARD)