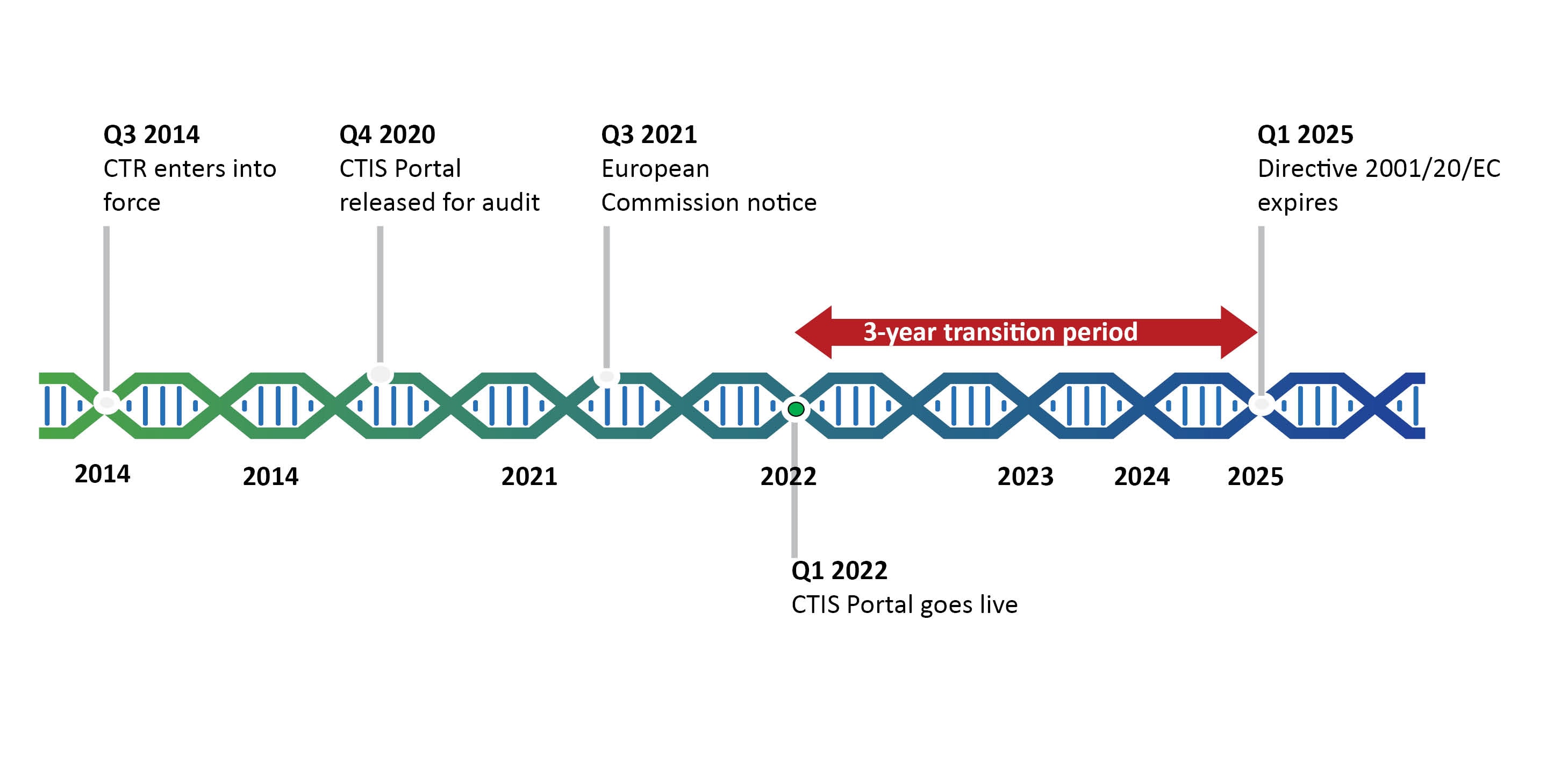
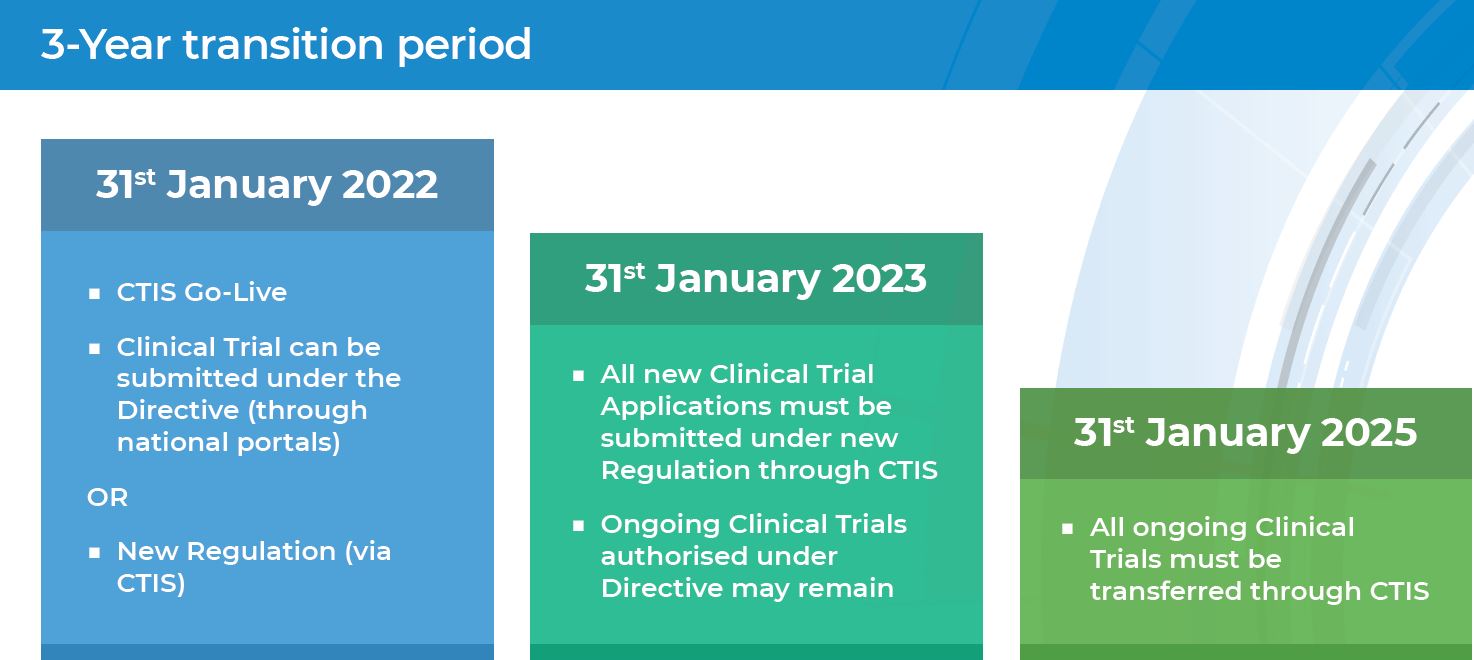
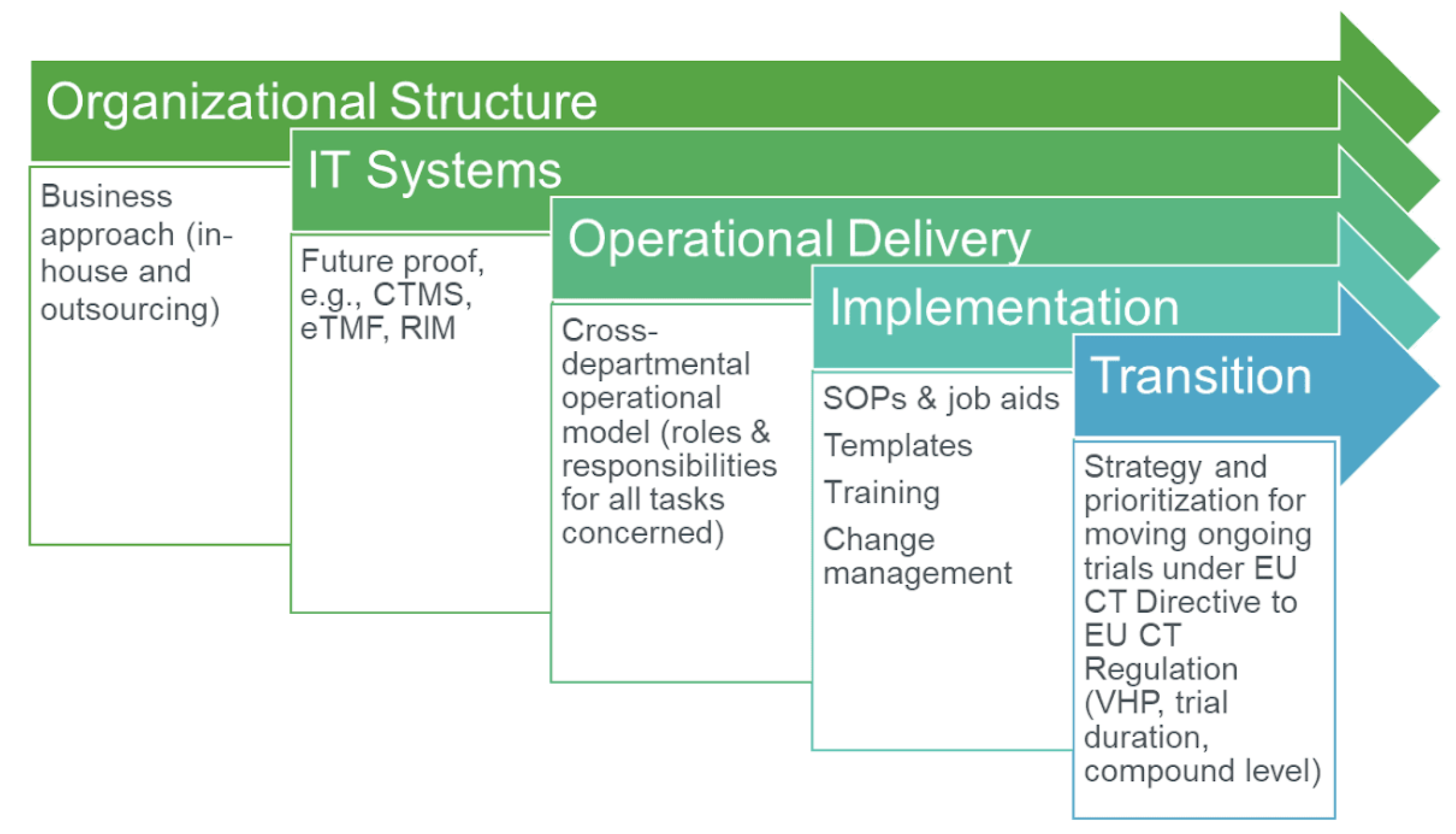
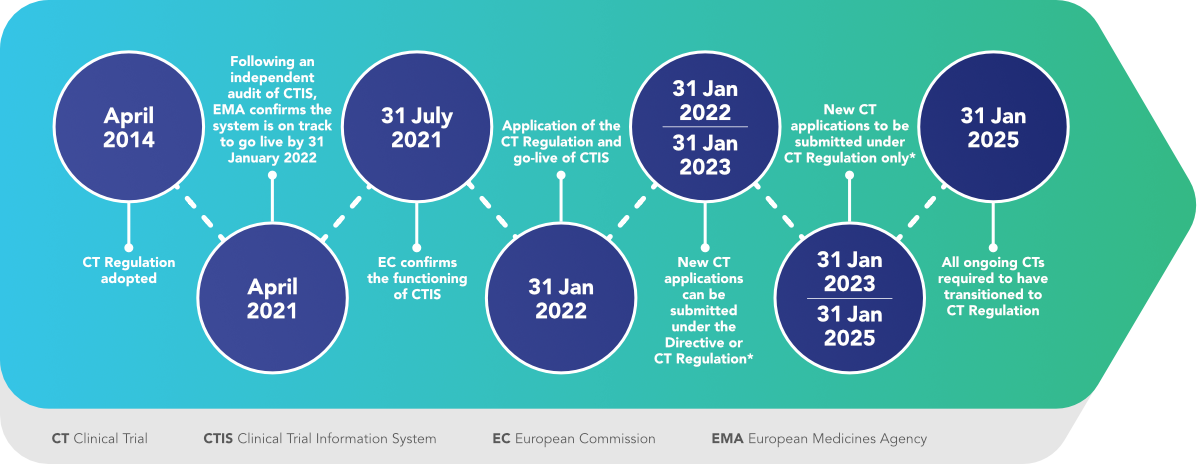
The Clinical Trials Directive: How Is It Affecting Europe's Noncommercial Research | PLOS Clinical Trials

Exploring the Impact of the New European Directive on the Pharmaceutical Industry - Clinical Trials Arena

Flow of clinical trials application according to 2001/20/EC Directive.... | Download Scientific Diagram

Directive 2001/20/EC : Clinical trials on medicinal products for human use - Free PDF download | M A N O X B L O G

Guide to Clinical Trials Authorised for Conduct under the Clinical Trials Directive (Council Directive 2001/20/EC) in Ireland